Research
A central tenet of our research is the development of small molecule tools to better understand neurotransmitter signaling. Our current efforts focus on programs for the kappa opioid, dopamine and sigma receptors. Each is a highly collaborative effort in concert with screening facilities and pharmacology experts, which has led to the identification of selective modulators for the individual receptors or receptor subtypes. Of particular interest toward our overarching goal is the recent revelation that CNS receptors are capable of biased activation (i.e. preference for either the G protein or βarrestin pathways). We have discovered G protein biased ligands for the kappa opioid receptor and have ongoing efforts to identify promising biased ligands for dopamine receptors. These biased ligands serve as molecular tools to study the mechanistic details, behavioral effects and therapeutic potential of biased activation. A related area of high interest is the identification of allosteric modulators, with our efforts on this front focused on individual dopamine receptor subtypes. Overall, the identification and characterization of selective molecular tools will greatly aid the investigation into the nuanced regulation and interconnectivity of neurotransmitter signaling.
Other research efforts are aimed at developing new approaches for the treatment of unmet medical needs. For example, while much progress has been made targeting primary tumor sites certain types of cancer still present disproportionately high mortality rates. Pancreatic cancer has a mortality rate of over 90%, almost exclusively through metastasis of the primary tumor. We have discovered an effective antimetastatic agent that reduces the prevalence of the perinucleoar compartment, a phenotypic indicator of metastatic potential. The compound has demonstrated in vivo efficacy in preventing or reducing the metastatic transformation of tumor grafts. Current efforts are directed toward advancing the promising therapeutic potential and elucidating the mechanism of action for this class of compounds.
Allosteric Dopamine Receptor Ligands
Dopamine receptors (DARs) comprise five distinct receptor subtypes that are responsible for controlling a wide range of physiological functions related to mood, movement and reward. Dysregulation of dopamine signaling or an imbalance in dopamine levels is, at least in part, the underlying cause of many serious central nervous system diseases. Of particular relevance in this proposal are Parkins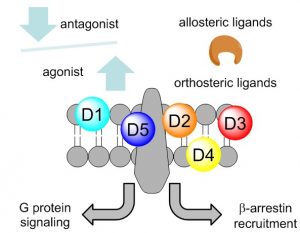 on’s disease, schizophrenia, Alzheimer’s disease and addiction. Dopamine is the endogenous neurotransmitter utilized by all receptor subtypes and the dopamine binding site is highly conserved across all dopamine receptors. While endogenous dopamine is spatially sequestered in neuronal cells, a drug discovery strategy targeting the dopamine binding site has led to small molecule therapeutics possessing low selectivity, and often therapeutically limiting side effects. Small molecules that interact with the specific DAR subtypes at alternative (allosteric) sites should lead to improved agents with high efficacy and fewer off-target effects. As the physiological effects associated with individual DARs is still not well understood, highly selective modulators of distinct DAR subtypes would provide valuable chemical tools for elucidating the downstream consequences of receptor activation. In collaboration with the pharmacology laboratory of David Sibley, we are working to optimize hit molecules from an HTS campaign aimed at the discovery of such allosteric dopamine modulators. More details on our current targets of interest can be found in the Publications tab.
on’s disease, schizophrenia, Alzheimer’s disease and addiction. Dopamine is the endogenous neurotransmitter utilized by all receptor subtypes and the dopamine binding site is highly conserved across all dopamine receptors. While endogenous dopamine is spatially sequestered in neuronal cells, a drug discovery strategy targeting the dopamine binding site has led to small molecule therapeutics possessing low selectivity, and often therapeutically limiting side effects. Small molecules that interact with the specific DAR subtypes at alternative (allosteric) sites should lead to improved agents with high efficacy and fewer off-target effects. As the physiological effects associated with individual DARs is still not well understood, highly selective modulators of distinct DAR subtypes would provide valuable chemical tools for elucidating the downstream consequences of receptor activation. In collaboration with the pharmacology laboratory of David Sibley, we are working to optimize hit molecules from an HTS campaign aimed at the discovery of such allosteric dopamine modulators. More details on our current targets of interest can be found in the Publications tab.
Selective Inhibition of Metastasis
The mortality of certain cancer subtypes remains disproportionately high due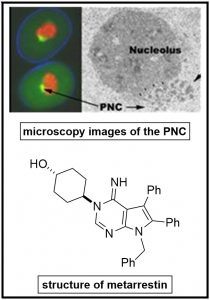 to the lack of effective treatment against metastasis, the primary cause of death in these patients, a consequence of the complex nature of metastasis and unclear understanding of the key metastasis mechanisms. Although targeting single genes or gene products has helped deter cancer growth, it falls short in treating metastatic conditions. To address this unmet need, we, in a joint effort with Sui Huang (Northwestern University) and NCATS (NIH), have developed an alternative approach, in which we used a complex cytological maker as a surrogate marker for metastatic behavior to better reflect the complex mechanisms of metastasis. We found perinucleolar compartment (PNC) formation closely associates with the metastatic potential of cancer cells in vitro and in vivo. Reduction of PNC prevalence was used to screen for compounds that interfere with cellular mechanisms essential for the metastatic capability of cancer cells. Hit optimization generated metarrestin, a first in class compound that disrupts the PNC with an IC50 around 300 nM. Metarrestin effectively inhibits metastasis in three murine models of human cancers (pancreatic, prostate and breast cancers) without significant adverse effects in treated animals. The selective metastatic inhibition makes metarrestin a useful molecular probe and lead chemotherapeutic candidate. Metarrestin recently began a first-in-human Phase I clinical trial for treatment-resistant cancer patients (NCT04222413). Our current interest in PNC inhibition is three-fold:
to the lack of effective treatment against metastasis, the primary cause of death in these patients, a consequence of the complex nature of metastasis and unclear understanding of the key metastasis mechanisms. Although targeting single genes or gene products has helped deter cancer growth, it falls short in treating metastatic conditions. To address this unmet need, we, in a joint effort with Sui Huang (Northwestern University) and NCATS (NIH), have developed an alternative approach, in which we used a complex cytological maker as a surrogate marker for metastatic behavior to better reflect the complex mechanisms of metastasis. We found perinucleolar compartment (PNC) formation closely associates with the metastatic potential of cancer cells in vitro and in vivo. Reduction of PNC prevalence was used to screen for compounds that interfere with cellular mechanisms essential for the metastatic capability of cancer cells. Hit optimization generated metarrestin, a first in class compound that disrupts the PNC with an IC50 around 300 nM. Metarrestin effectively inhibits metastasis in three murine models of human cancers (pancreatic, prostate and breast cancers) without significant adverse effects in treated animals. The selective metastatic inhibition makes metarrestin a useful molecular probe and lead chemotherapeutic candidate. Metarrestin recently began a first-in-human Phase I clinical trial for treatment-resistant cancer patients (NCT04222413). Our current interest in PNC inhibition is three-fold:
1) Use metarrestin and derivatives as chemical tools to elucidate the molecular pathways of metarrestin’s striking antimetastatic activity, potentially uncover universal key metastatic mechanisms and better understand the functional relevance of PNCs.
2) Further explore metarrestin analogues to obtain even more potent PNC inhibitors. Improved metarrestin analogs would serve as improved leads for preclinical development and be used to examine the cellular pathways impacted in comparison with metarrestin to search for cellular targets or pathways important for PNCs and, potentially, for metastasis.
3) Develop chemically distinct PNC modulators, which could possess different polypharmacology as such an array of molecular tools would be useful to elucidate the molecular mechanism linking the PNC and metastasis. The development of new probes would provide complementary chemical tools to dissect the functional connection between PNC reduction and metastatic behavior, and afford new therapeutic leads.
We are highly grateful for the past and present support of our research: